BEXSERO OFFERS DEMONSTRATED IMMUNE RESPONSE TO DIVERSE DISEASE-CAUSING MenB STRAINS AND 4 INDICATOR STRAINS
Only BEXSERO measures the breadth of immune response against a panel of 110 diverse disease-causing N. meningitidis serogroup B strains.1,2
hSBA=human serum bactericidal activity; enc-hSBA=endogenous complement hSBA.
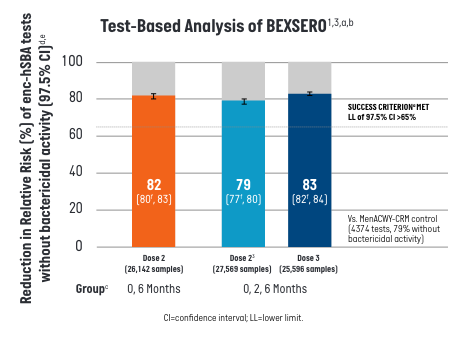
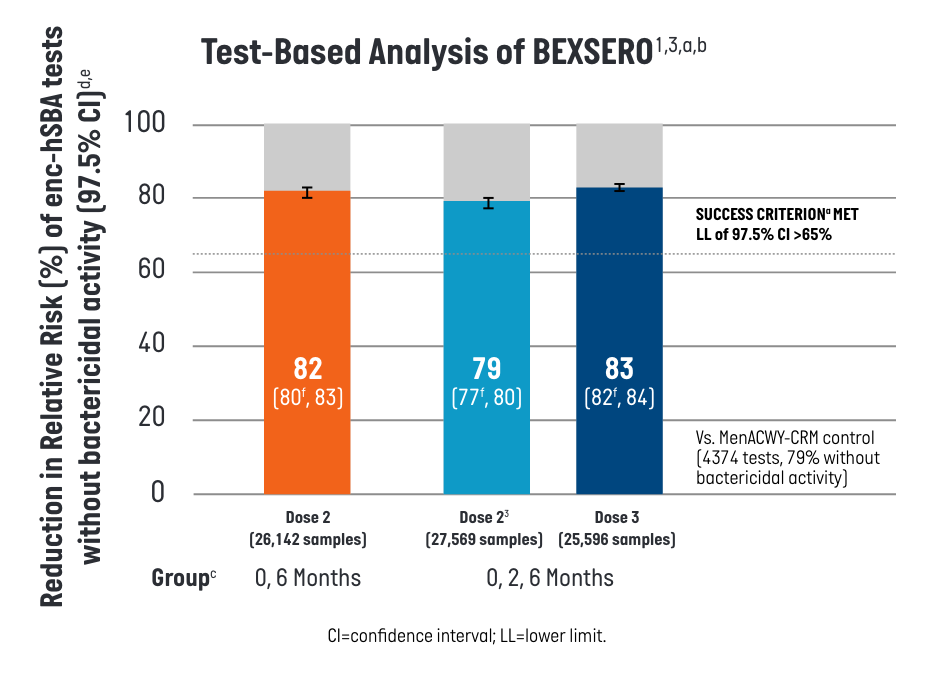
Participants who received BEXSERO on a 2-dose (0-, 6-month) or 3-dose (0-, 2-, 6-month) schedule showed a >80% relative risk (RR) reduction against a panel of 110 diverse disease-causing MenB strains.1
- aEach test qualitatively assessed (yes/no) the bactericidal activity of one participant’s serum against one of the 110 U.S. meningococcal serogroup B strains. Each participant’s serum was tested against a maximum of 35 strains randomly selected from the 110-strain panel.
- bPer Protocol Set includes all participants in the Full Analysis Set minus participants with protocol deviations that lead to exclusion from the Per Protocol Set.
- cenc-hSBA responses were measured one month after the second dose of BEXSERO using the 0-, 6-month schedule, 1 month after the second and third doses of BEXSERO using the 0-, 2-, 6- month schedule, and 1 month after the single dose of MENVEO.
- dReduction in Relative Risk of enc-hSBA tests without bactericidal activity is defined as 1 - RR = (1 - percentage of samples without bactericidal serum activity measured by enc-hSBA in the BEXSERO group / percentage of samples without bactericidal serum activity in the MENVEO group) x 100%.
- eThe Relative Risk and corresponding confidence intervals are estimated using a generalized linear model where treatment group and randomization factors were modeled as independent variables.
- fPredefined criterion (lower limit of the 2-sided 97.5% CI >65%) met.
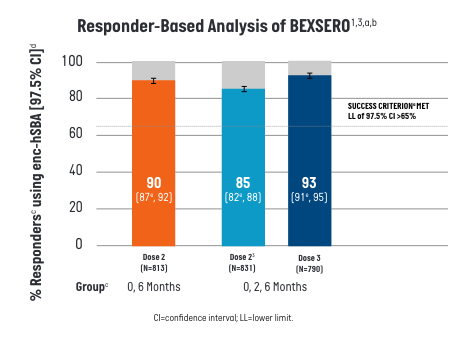
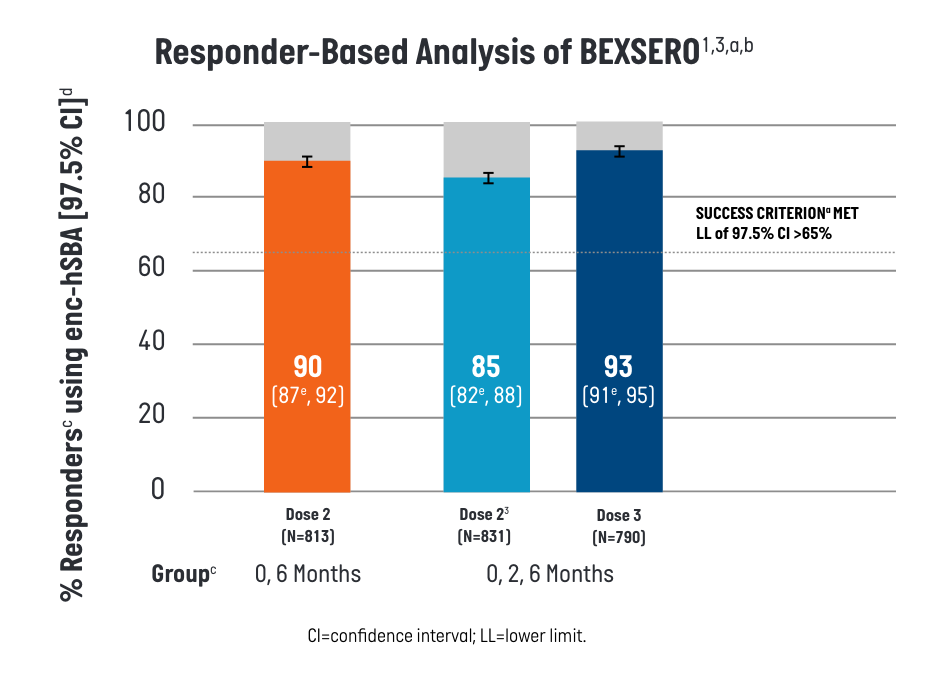
In the responder-based analysis, ≥90% of participants who received BEXSERO on a 2-dose (0-, 6-month) or 3-dose (0-, 2-, 6-month) schedule killed ≥70% of tested strains, meeting the pre-defined success criterion.1
- aEach participant’s serum was tested for bactericidal activity (yes/no) against a maximum of 35 strains randomly selected from the 110-strain panel.
- bFull Analysis Set includes all participants who received at least 1 dose of the study treatment and have post-vaccination immunogenicity data.
- cenc-hSBA responses were measured one month after the second dose of BEXSERO using the 0-, 6-month schedule and one month after the second and third doses of BEXSERO using the 0-, 2-, 6- month schedule.
- d% Responders is defined as percentages of participants whose serum kills ≥70% of strains tested using enc-hSBA.
- ePredefined criterion (lower limit of the 2-sided 97.5% CI >65%) met. CI calculated using Clopper-Pearson method.
Study design: The breadth of immune response elicited by BEXSERO was evaluated in a phase 3, randomized, controlled, observer-blind trial (NCT04502693) conducted in 7 countries in participants aged 10 through 25 years.
Participants received BEXSERO using either a 0-, 2-, 6-month schedule (n=897) or a 0-, 6-month schedule (n=906). A single dose of MENVEO was administered as a control (n=178).
The test-based analysis evaluated the reduction in relative risk of enc-hSBA tests without bactericidal activity against meningococcal serogroup B strains following BEXSERO as compared with a single dose of MENVEO as the control.
The responder-based analysis assessed the percentage of participants whose sera killed ≥70% of the tested strains in participants who received BEXSERO.
The predefined success criterion for both endpoints (LL of the 2-sided 97.5% CI >65%) was met for each of the BEXSERO schedules.1
Demonstrated immunogenicity against the 4 indicator strains, one for each of the antigenic components contained in BEXSERO: fHbp, NadA, NHBA, and OMV
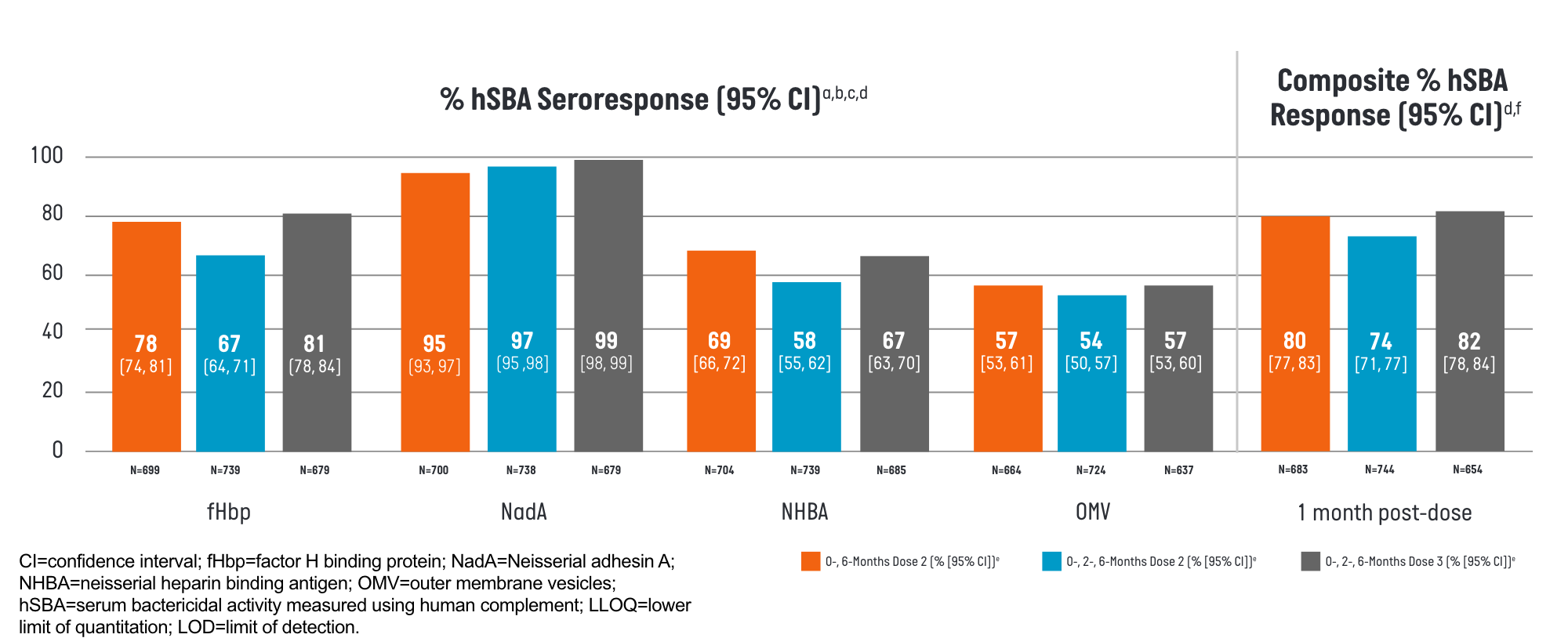
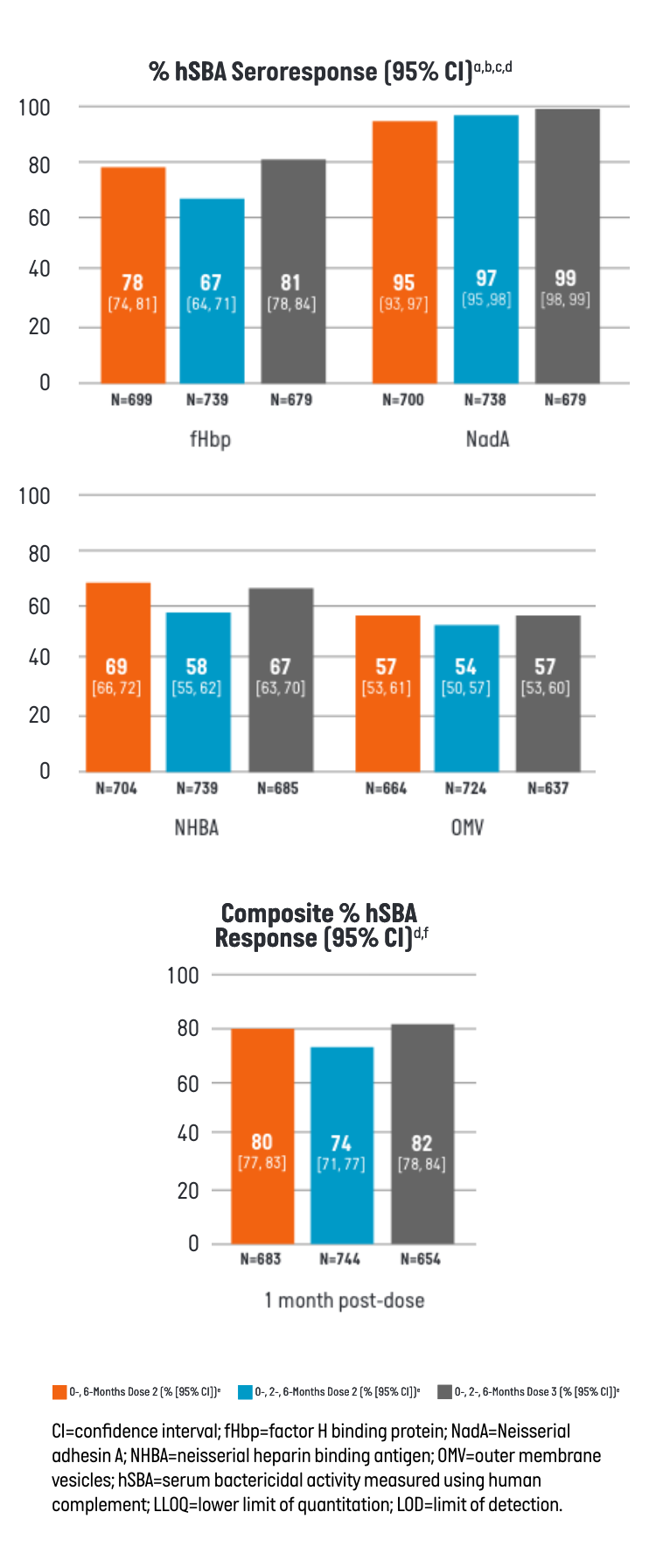
BEXSERO demonstrated an immune response (% hSBA seroresponse), measured for 4 indicator strains, each representing one of its 4 distinct antigenic components as well as composite % hSBA seroresponse.1
- aFull Analysis Set includes all participants who received at least 1 dose of the study treatment and have post-vaccination immunogenicity data.
- bSeroresponse is defined as: a post-vaccination hSBA titer at least 4-fold the LOD or ≥LLOQ, whichever is greater, for participants with pre-vaccination hSBA titer <LOD, a post-vaccination hSBA titer at least 4-fold the LLOQ for participants with pre-vaccination hSBA titer ≥LOD and <LLOQ, and a post-vaccination hSBA at least 4-fold the pre-vaccination hSBA titer for participants with pre-vaccination hSBA titer ≥LLOQ.
- cAntigen (indicator strain) = fHbp (M14459), NadA (96217), NHBA (M13520), OMV (NZ98/254).
- dLOD = 4 for fHbp (M14459); 6 for NadA (96217); 4 for NHBA (M13520); 4 for OMV (NZ98/254). LLOQ = 5 for fHbp (M14459); 14 for NadA (96217); 6 for NHBA (M13520); 6 for OMV (NZ98/254).
- eCI calculated using Clopper-Pearson method.
- fComposite hSBA Response means hSBA ≥LLOQ for all 4 Meningococcal B indicator strains.
Study design: lmmunogenicity was assessed in the phase 3 trial (NCT04502693) in participants aged 10 through 25 years who received BEXSERO using either a 0-, 6-month or a 0-, 2-, 6-month schedule. The seroresponses were measured 1 month after the second dose for both schedules and after the third dose for the 0-, 2-, 6-month schedule.1
Vaccination may not protect all recipients.