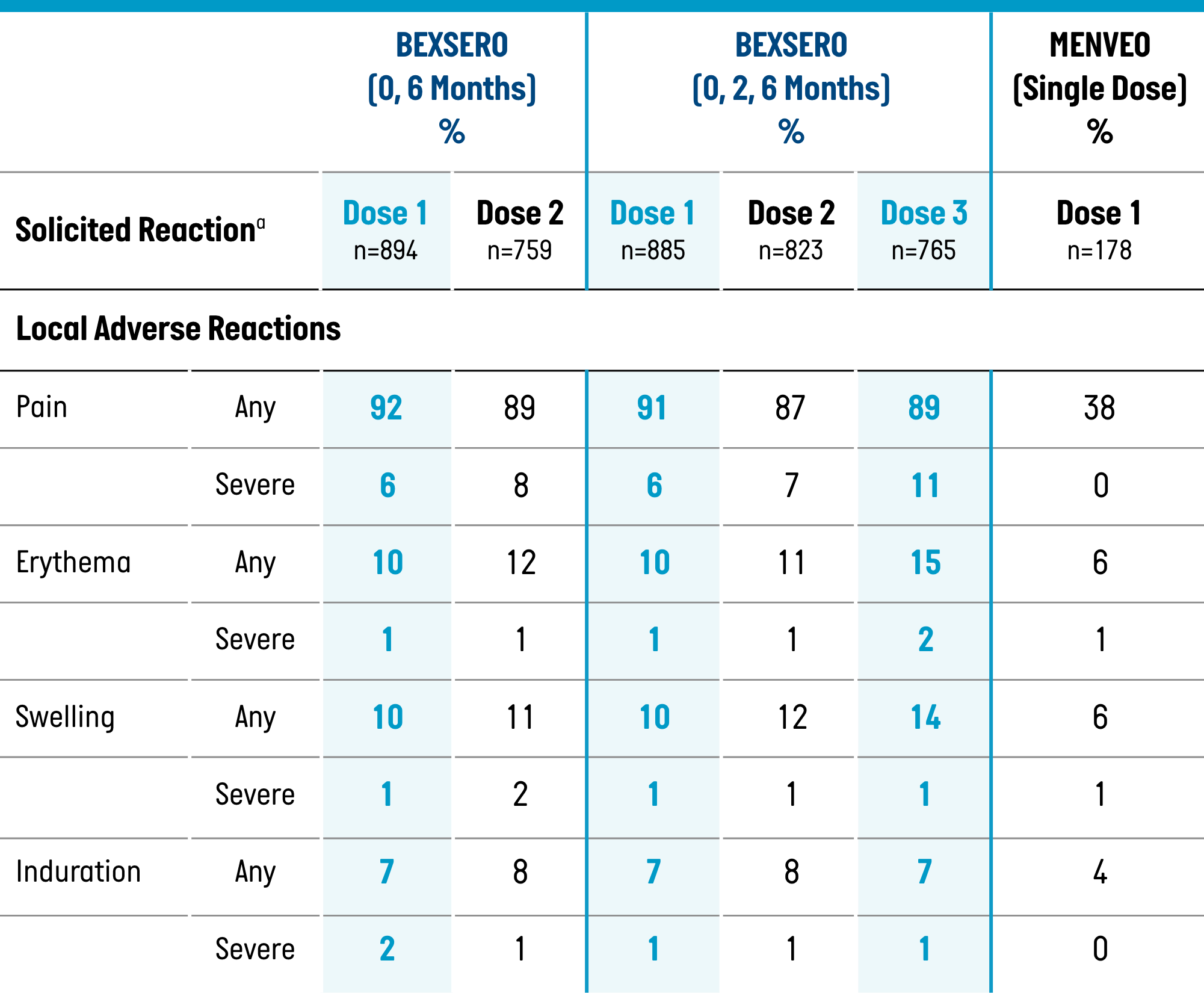
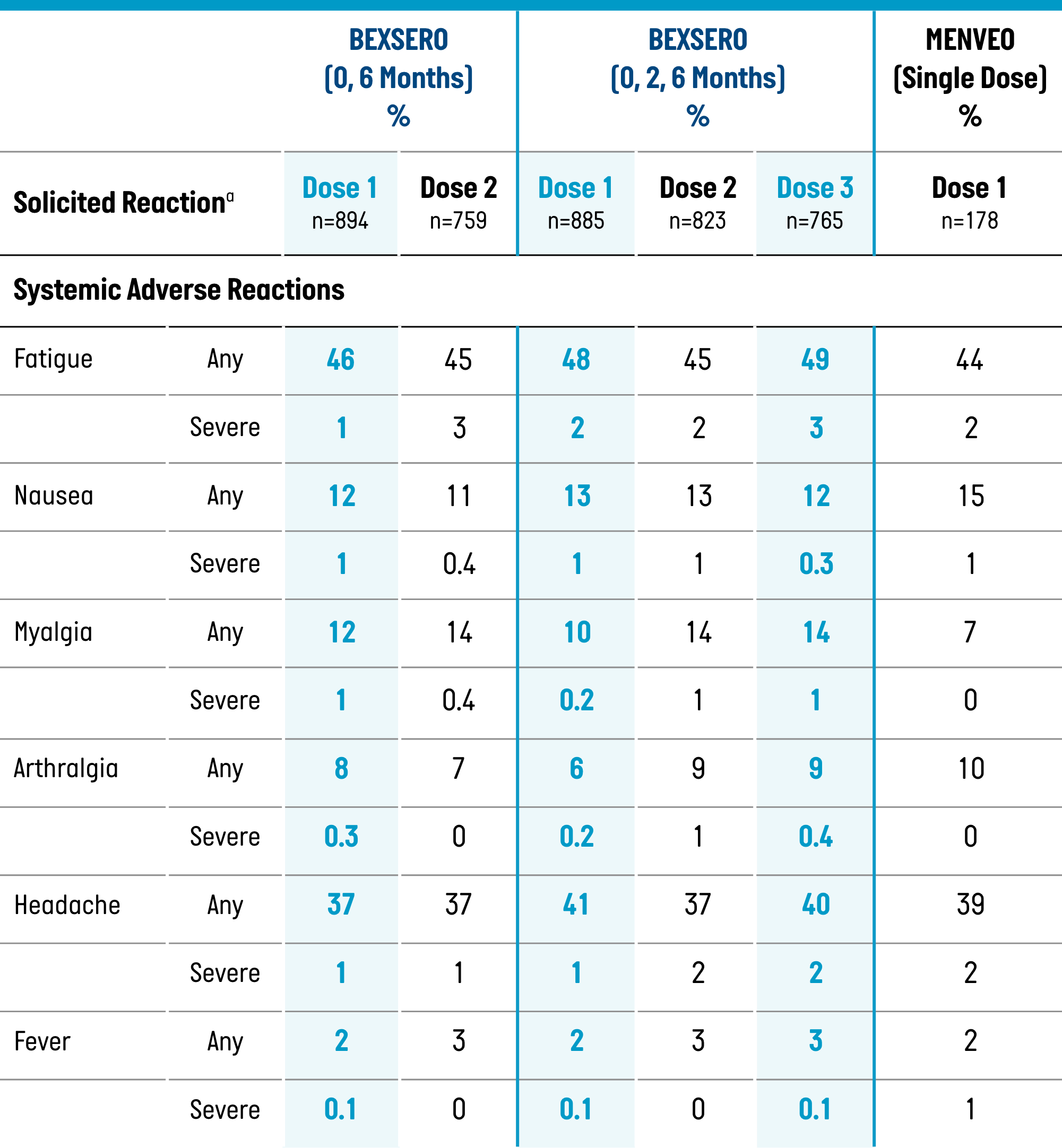
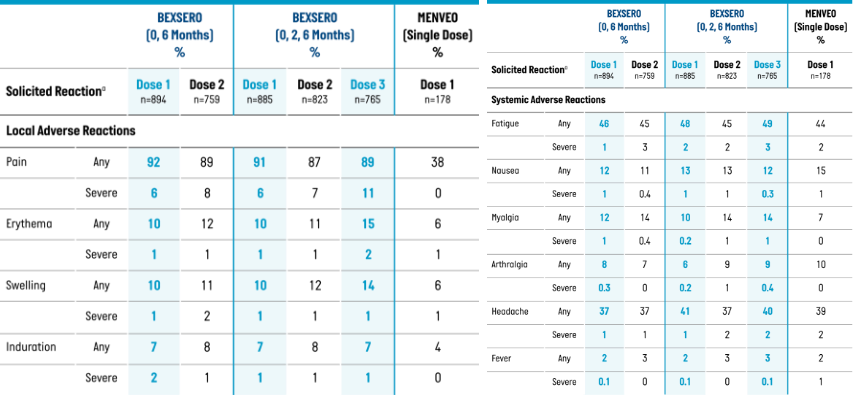
Percentage of Participants Aged 10 Through 25 Years Reporting Solicited Local and Systemic Adverse Reactions Within 7 Days of BEXSERO or MENVEO, by Dose (Solicited Safety Set, Study 1)1
| Dose 1: Any (Severe) | Dose 2†: Any (Severe) | |||
|---|---|---|---|---|
| BEXSERO (N=110-114) |
SALINE PLACEBO (N=94-96) |
BEXSERO (N=107-109) |
MENVEO (N=90-92) |
|
Solicited Local Adverse Reactions
Severe=unable to perform normal daily activities
| Pain | 90% (20%) | 27% (2%) | 83% (29%) | 43% (8%) |
| Erythema: Any (≥1 mm) | 50% | 13% | 45% | 26% |
| >100 mm | 0% | 0% | 0% | 2% |
| Induration: Any (≥1 mm) | 32% | 10% | 28% | 23% |
| >100 mm | 0% | 0% | 0% | 2% |
Solicited Systemic Adverse Reactions
| Fatigue | 37% (4%) | 22% (0%) | 35% (6%) | 20% (2%) |
| Nausea | 19% (4%) | 4% (0%) | 18% (4%) | 4% (0%) |
| Myalgia | 49% (12%) | 26% (1%) | 48% (13%) | 25% (4%) |
| Arthralgia | 13% (2%) | 4% (0%) | 16% (2%) | 4% (0%) |
| Headache | 33% (4%) | 20% (1%) | 34% (6%) | 23% (3%) |
| Fever (≥38 °C) | 1% | 1% | 5% | 0% |
| Dose 1: Any (Severe) | ||
|---|---|---|
| BEXSERO (N=110-114) |
SALINE PLACEBO (N=94-96) |
|
Solicited Local Adverse Reactions
Severe=unable to perform normal daily activities
| Pain | 90% (20%) | 27% (2%) |
| Erythema: Any (≥1 mm) | 50% | 13% |
| >100 mm | 0% | 0% |
| Induration: Any (≥1 mm) | 32% | 10% |
| >100 mm | 0% | 0% |
Solicited Systemic Adverse Reactions
| Fatigue | 37% (4%) | 22% (0%) |
| Nausea | 19% (4%) | 4% (0%) |
| Myalgia | 49% (12%) | 26% (1%) |
| Arthralgia | 13% (2%) | 4% (0%) |
| Headache | 33% (4%) | 20% (1%) |
| Fever (≥38 °C) | 1% | 1% |
| Dose 2†: Any (Severe) | ||
|---|---|---|
| BEXSERO (N=107-109) |
MENVEO (N=90-92) |
|
Solicited Local Adverse Reactions
Severe=unable to perform normal daily activities
| Pain | 83% (29%) | 43% (8%) |
| Erythema: Any (≥1 mm) | 45% | 26% |
| >100 mm | 0% | 2% |
| Induration: Any (≥1 mm) | 28% | 23% |
| >100 mm | 0% | 2% |
Solicited Systemic Adverse Reactions
| Fatigue | 35% (6%) | 20% (2%) |
| Nausea | 18% (4%) | 4% (0%) |
| Myalgia | 48% (13%) | 25% (4%) |
| Arthralgia | 16% (2%) | 4% (0%) |
| Headache | 34% (6%) | 23% (3%) |
| Fever (≥38 °C) | 5% | 0% |
a
Erythema, swelling, and induration: Any (≥25 mm); Severe (>100 mm). Pain, fatigue, nausea, myalgia, arthralgia, headache: Any includes Mild (transient with no limitation in normal daily activity), Moderate (some limitation in normal daily activity), and Severe (unable to perform normal, daily activity). Fever: Any (≥38.0 °C/100.4 °F); Severe (≥40.0 °C/104.0 °F).
Study design: The safety of BEXSERO was evaluated in 5 clinical studies in which a total of 4,861 participants aged 10 through 25 years received at least 1 dose of BEXSERO. In Study 1, conducted in the United States, Australia, Canada, Czech Republic, Estonia, Finland, and Turkey, 1,803 participants aged 10 through 25 years received at least 1 dose of BEXSERO either as a 0-, 6-month schedule (n=906) or a 0-, 2-, 6-month schedule (n=897). A single dose of MENVEO [Meningococcal (Groups A, C, Y and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine] was administered 2 months after the first dose of BEXSERO in the 0-, 6-month group and 1 month after the third dose in the 0-, 2-, 6-month group. In the control group, 178 participants received a single dose of MENVEO followed by 2 doses of BEXSERO administered 1 month apart.1
Vaccination may not protect all recipients.
CDC=Centers for Disease Control and Prevention.