These real-world data are designed to evaluate association among variables; causality cannot be established. Results are descriptive only.
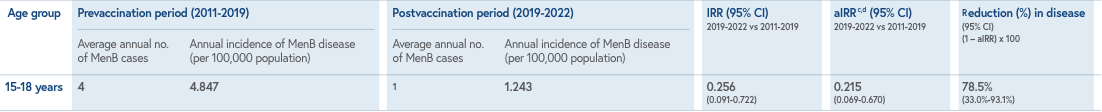
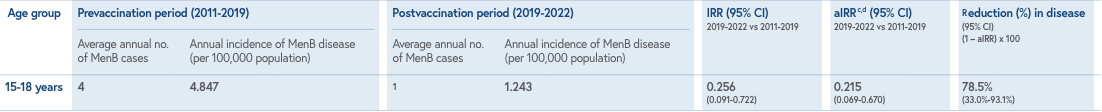
Study design: Observational, interrupted time-series analysis assessing vaccine impact (VI) of BEXSERO on invasive MenB disease incidence in adolescents 15-18 years of age in the state of South Australia.1,2 VI was estimated as the incidence rate ratio (IRR) and adjusted incidence rate ratio (aIRR), obtained by comparing the average annual incidence of MenB cases in the 3-year period following the start of the vaccination program (2019-2021) with the average incidence of MenB cases in the equivalent age cohort during the prevaccination program years (2011-2019).1
Study limitations: The study was conducted only in South Australia, where 1 of the 4 antigenic components (PorA) of the BEXSERO vaccine was closely matched to the circulating disease-causing isolate. The impact of the vaccine may differ for the US with different serogroup B isolates.1,3
Public health strategies implemented during the COVID-19 pandemic may have inadvertently contributed to reductions in MenB disease incidence during the second and third years of the program. These interventions, aimed against SARS-CoV-2 transmission, may have contributed to reductions in MenB disease incidence and impact in 2019-2020. The authors’ previous research showed the usual seasonal increase in N. meningitidis oropharyngeal carriage during this period of physical distancing policies. The VI analysis does not take into account vaccine uptake and might be vulnerable to confounding factors due to trends unrelated to the intervention.1
Bias may be introduced in time-series studies due to inadequately modeled time trends, or cyclical variations in disease, case detection, population characteristics, or introduction of concomitant interventions.3